- General
- Telehealth
- Career Trends
- Featured
- Legal
- News and Events
- Technology
- How To
- Procedures
- Training
- Allied Health Industry
- Allied Health Practitioners
- Employer News
- Candidate News
Recent Posts
Most Popular
Pfizer-BioNTech say low dose of its COVID-19 vaccine is safe and effective for kids 5-11
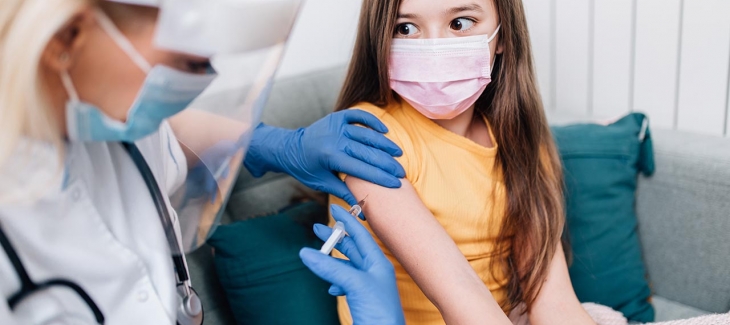
Pfizer-BioNTech's COVID-19 vaccine is safe and effective for children ages 5 to 11 at one-third the dose used in adolescents and adults, according to a new study from the companies.
Many parents have been eagerly awaiting a vaccine for children, who have returned to school amidst a national wave of COVID-19 cases. Cases of COVID-19 in children have jumped about 240% since July.
Children are less likely to become severely ill from COVID-19 than adults, but they can catch and pass on the virus and occasionally suffer serious disease and long-term consequences.
The Food and Drug Administration and Centers for Disease Control and Prevention will need to sign off on the vaccine before it becomes available to children, but government officials have promised to quickly review the data.
Authorization is likely to come within "a matter of weeks not months," the FDA's Dr. Peter Marks told USA TODAY recently.
"FDA is committed to going through those as quickly as we (can)," he said. "These data will not lay around when they come in."
The Pfizer-BioNTech vaccine, called Comirnaty, is now fully approved for use in adults and older teens, though still authorized only for emergency use in 12- to 15-year-olds.
"We are eager to extend the protection afforded by the vaccine to this younger population, subject to regulatory authorization, especially as we track the spread of the delta variant and the substantial threat it poses to children,” Albert Bourla, Pfizer's chairman and chief executive officer, said in a statement. "These trial results provide a strong foundation for seeking authorization of our vaccine for children 5 to 11 years old, and we plan to submit them to the FDA and other regulators with urgency.”
The adult dose of the Pfizer-BioNTech vaccine is 30 micrograms, while the companies propose a 10 microgram dose in children ages 5 to 11. As in adults, the vaccine would be given to children in two shots, delivered at least three weeks apart.
At this lower dose, the vaccine is safe for children, leading to the same types of mostly minor side effects seen in adolescents and young adults, according to the new research.
"Our objective was to generate and submit the data for school kids to regulatory authorities around the world before the winter season begins,” Dr. Uğur Şahin, CEO and co-founder of BioNTech, said in a prepared statement.
The study of 2,268 volunteers ages 5 to 11 showed they mounted the same type of strong immune response to the vaccine as teens and young adults. Because Comirnaty has already proven effective in older groups, the companies only had to show that the vaccine led to a similar immune response in children – rather than prove it prevented COVID-19 infections – which is why this study could be so much smaller than the 44,000-person trial in adults.
The companies are also studying their vaccine in children ages 2 to 5, and 6 months to age 2, but those trials are not yet complete. Younger children are being tested on a 3 microgram dose.
Participants in all three trials of children come from more than 90 locations in the United States, Finland, Poland and Spain, the companies said. Some had previously been infected with COVID-19, while others had not.
The other two companies with COVID-19 vaccines authorized for use in the United States, Moderna and Johnson & Johnson, are also studying their vaccines in children, but have not yet completed their research.
Until a vaccine is approved for children, public health experts, including the FDA's Marks, have said that the best thing parents can do to protect their children is to get vaccinated themselves. Children should be surrounded by vaccinated adults and should wear masks while indoors in public.
"No parents should lose a child to an infectious disease that can be prevented by a vaccine," Marks said.
Elizabeth Weise contributed to this report.
Contact Karen Weintraub at kweintraub@usatoday.com.
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.
This article originally appeared on USA TODAY: COVID vaccine for kids 5-11: Pfizer says low dose safe, effective








Comments